Health
Find all information around assortment policies for the Health category here.
Health and nutrition claims
Claims can never be misleading, confusing or ambiguous and must always be understandable to consumers. Before you can use a health or nutrition claim, it must be assessed, approved and listed in the European Claims Register. Claims must be scientifically substantiated, and the indication of nutritional values is mandatory.
When placing health claims on the product picture or in the description, take into account the guidelines of the European Claims Regulation 655/2013 and make sure these claims comply with the applicable laws and regulations.
It is not allowed to make medical claims.
Coronavirus
To avoid possible deception, it is not allowed to refer to coronavirus in the product picture, title and description.
Check whether your claim is allowed with this useful database:
Precursors for explosives
European regulation on explosives precursors
Home-explosives are weapons commonly used by terrorists and other criminals. This is due to the accessibility of some raw materials for explosives. The availability of these substances is restricted by European regulations. As of February 1, 2021, the new European Regulation 2019/1148 applies for explosives precursors. This may have implications for you as an entrepreneur and partner of bol.
The implications for you as an entrepreneur
The regulation brings new obligations for sellers of assortment that may contain raw materials for explosives, so possibly also for you as a business owner. These obligations are described in European Regulation 2019/1148. You can read more information on the website of the NCTV (note: this website has not yet been updated to the new regulation). Bol, as an online platform, is legally obliged under the Regulation, among other things, to report suspicious transactions, disappearance and theft of items containing certain raw materials to the Suspicious Transactions Chemicals Disclosure Office. Please note that this reporting obligation does not only apply to online platforms like bol, but also to you as an entrepreneur.
The implications for your offer on bol – provide additional information
Bol will only allow the sales of items in which concentrations of the specific raw materials are below the limit for (license-free) sales as specified in the regulation. To make this possible, from February 1, 2021, it will be compulsory at bol to fill in the concentrations of the specific raw materials at product attributes. If you do not fill in this information before 1 February, the relevant article will be taken offline. This will also happen if the concentrations of the specific raw materials are above the limit mentioned above. The annexes of the European Regulation 2019/1148 describe the specific limit values of the raw materials. You can find the regulation here.
Which product attributes are mandatory?
Below you can find the raw materials that are mandatory to add to your product attributes, depending on the type of assortment:
These items, among others, may contain these raw materials:
CE & ECE marking
The so-called CE & ECE markings indicate that your items comply with safety, health and environmental requirements. For most items marketed in the European Union (EU), this marking is even mandatory.
Is CE marking also mandatory for you?
CE marking is mandatory for items in the following product groups (please note that this does not mean you are allowed to sell all these items via bol):
- Explosives for civilian use
- Pyrotechnic articles
- Construction products
- Toys
- Personal protective equipment
- Electrical, electronic and energy-related items
- Medical devices (please note that these are subject to specific guidelines you need to comply with in addition to the CE markings. You can read about it in Medical Devices Policy)
- Measuring and weighing equipment
- Machinery and related items
- Transport equipment
Is the ECE marking also mandatory for you?
An ECE marking is mandatory for items in the following product groups (please note, this does not mean that you can sell all these items via bol):
- Car seats
- Car lamps
- Helmets
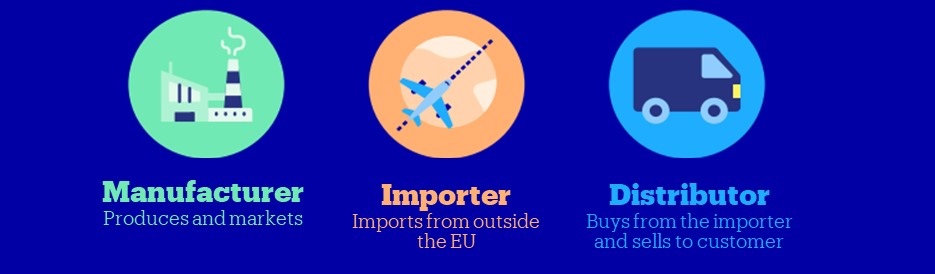
Which responsibilities you have regarding the CE marking depends on your role – i.e. whether you are the importer, distributor or manufacturer. It is your own responsibility to determine whether the articles you sell require CE marking. Use this overview from the RVO to check whether CE marking is mandatory for your item.
Take the following actions to ensure that your product range complies with CE marking:
- Complete the product information ‘CE marking’. You can find this below mandatory information in the seller dashboard. Via a dropdown menu you can indicate that the CE marking is ‘visible’ (on your item, on the packaging of your item or on a manual of your item). Should your item still not be covered by CE regulations, you can select ‘not applicable’ here. We will check this product information. For current offer it is not obliged to upload a photo.
- For new product ranges (this means offering a new item for which no product information is yet available), you must upload a photo (JPEG or PDF file type) of the CE marking as applied to the item. If this is not technically possible, a photo of the CE marking on the packaging or on the instruction manual will suffice. Instructions and examples for adding CE marking can be found here.
- The trade name and contact address of the manufacturer or importer in Europe:
For existing and new ranges, we ask you to fill in the text attribute ‘Trade name and contact address of manufacturer or importer in EU‘ under optional information in the seller dashboard. You should fill in the name and contact address of the manufacturer or importer in EU. The information entered will not be visible on bol, nor will we search for the purchase channel. You hereby declare that the information entered is also visible/present on the product or packaging.
Please take the following actions to ensure your range is compliant with the ECE marking:
- We ask you to fill in the product information ‘ECE marking’ from 24 August 2023 (under optional information in the seller dashboard). From 1 March 2024, this can be found under compulsory information in the seller dashboard. Here you can indicate via a dropdown menu that the ECE marking is ‘visible’ (on your item, on the packaging of your item or on a manual of your item). Should your item still not be covered by ECE regulations, you can select ‘not applicable’ here. We will check this product information. For existing offer, it is not required to upload a photo.
- For new assortment (this means offering a new article for which no product information is available yet), from 1 March 2024 you have to upload a photo (JPEG or PDF file type) of the ECE marking as applied to the item. If this is not technically possible, a photo of the ECE marking on the packaging or on the instruction manual will suffice. To add the ECE marking, please use the instructions for CE marking, which can be found here.
- The trade name and contact address of the manufacturer or importer in Europe:
For existing and new offer, we ask you to fill in the text attribute ‘Handelsnaam en contactadres van fabrikant of importeur in EU’, to be filled in under optional information in the seller dashboard. From 1 March 2024, this will be mandatory. Here you must then fill in the name and contact address of the manufacturer or importer in the EU. The information entered will not be visible on bol, nor will we search for the purchase channel. You hereby declare that the information entered is also visible/present on the item or packaging.
- If you sell an item from a country outside the EU and sell it under that manufacturer’s brand name, then you are an importer, with the accompanying responsibilities.
- Do you sell an item you bought from a party within the EU? For example, from an importer, a European manufacturer or another distributor? Then you are a distributor.
We will check the product information for completeness and accuracy.
-
- If the item is a toy and has safety warnings, add these in the textual product information below ‘Veiligheidswaarschuwingen’ (Safety warnings).
- Make sure you have the documentation and the declaration of conformity (conformity statement) accompanying the item available. Both bol and the authorities could ask for these.
Should you need help for the above, we recommend you consult the following resources:
- The website of the RFO
- European Commission website
- Our service partners who can give you advice
- Our policy on the Partnerplatform
How to use the CE mark?
You can recognise the CE marking by the familiar CE mark. It must be affixed to the product or the attached data plate. If this is not technically practicable, you may affix the CE marking to the product packaging or to an accompanying document (you can find the appropriate image here).
What if you do not fulfil this obligation?
The (safety) rules are set by law – and so you need to be able to prove that you have the necessary CE mark(s). If not, you are breaking the law. If you do not comply with our policy, your product will be taken offline. We do this to prevent customers from being put at risk by potentially unsafe items.
Want to find out more?
To meet all product safety requirements, our service partners are happy to help you on your way. They also offer advice and guidance on testing, certification, marking, quality assurance, labelling, manual writing and packaging design. Read more about our service partners and how they can help you on our product safety Partnerplatform.
Tobacco & Drugs
Bol chooses not to encourage the use of tobacco or soft and hard drugs; therefore, it is not allowed to sell the following products on bol:
- Tobacco and other smoking products covered by the Tobacco and Tobacco Products Act. In addition, bol goes beyond the law as it is neither allowed to sell cigarette makers or accessories with aroma, such as aroma cards, aroma filters, aroma cigarette papers and aroma liquids.
- Products covered by the Opium Act.
- E-cigarettes, vaporisers and any other products that can be used to consume nicotine (or a nicotine-free substance), which release vapour through a mouthpiece. Parts of and accessories for these products, including refills, cartridges, tanks and kits, are also prohibited from carrying on bol.
- Pipes, bongs, cannabis pipes and bongs. Parts of and accessories for these products are also prohibited from carrying on bol.
- Items associated with the production and consumption of soft and hard drugs. These include (electric) grinders, joint casings & rollers, rolling trays & boxes and cannabis seeds.
- Whipped cream cartridges.
However, the following items are allowed:
- Ashtrays
- Lighters
- CBD products, provided they comply with applicable laws and regulations in the Netherlands and Belgium
The following articles are allowed, provided they do not contain references to cannabis:
- Electric spice grinders
- Cigarette & cigarette boxes
Currently, selling rolling papers & tips is still allowed provided they do not contain references to cannabis. From July 1, 2024, these may no longer be sold on bol because we do not want to encourage the use of tobacco or soft and hard drugs.
Smoking accessories are also not allowed to display pictures of cigarettes or cigars.
Medicines
There are different types of medicines. Generally Available (GS) medicines can be sold via bol provided you are registered as an online provider of Generally Available medicines. Every medicine offered for sale (online) in the EU has a marketing authorisation issued by the EMA (Europe) or the CBG (Netherlands). These authorised medicines for human use and their marketing authorisation can be found in the Medicines Information Database.
It is not possible to sell Prescription-only (UR) medicines, Pharmacy-only (UA) medicines and Pharmacy-only (UAD) medicines via bol.
Teeth whitening products
The safety and health of our customers is paramount. It is important not to exceed the legal maximum of hydrogen peroxide in teeth whitening products. Therefore, it is mandatory to indicate the percentage of hydrogen peroxide (H202) in the product information attribute ‘percentage of hydrogen peroxide’ for that item.
Melatonin
A legal maximum daily dosage for the free sale of melatonin (supplements) has been established: products leading to a daily dosage of 0.3 milligrams or more of melatonin are considered by the Health Care and Youth Inspectorate (IGJ) to be a medicine. As these products fall under the Medicines Act, these products are (for now) unsuitable for sale on bol.com. Melatonin (supplements) that have a maximum daily dose lower than 0.3 milligrams are still allowed: the IGJ considers these to be food supplements.
Plant materials
It is not allowed to offer items with materials of plant species threatened with extinction through the platform. These species are on the lists of CITES. If you have articles containing protected plants, you are not allowed to offer them via bol. Not even if you have a valid permit, declaration or license to do so.
For example, the following materials are not allowed:
- Red coral (blood coral)
- Rosewood
> Discover protected plant species with the CITES checklist (English & French)
Food supplements (FAVV) – BE
Do you want to offer food supplements for the Belgian market via bol.com? To do so, your organisation must be registered with the FAVV with the relevant distribution authorisation.
Guidelines in Belgium
All organisations operating in the food chain in Belgium and having a warehouse in Belgium must be known to the Food Agency, and therefore registered. This includes the offering of notified food supplements, so in order to sell these items, your organisation must be registered with the FASFC.
Notified dietary supplements
Partners who want to sell food supplements in Belgium may only sell listed food supplements. Should you wish to offer dietary supplement articles that are currently not yet listed as notified with us, you can send a list of these EANs including notification numbers to gezondheid@bol.com. This list will then be checked and opened if the notifications are in order. It may possibly take 2 weeks for your request to be processed as there is a lot of manual work involved in this checking process.
FASFC registration
You can register your organisation with the FASFC via the following link: http://www.favv.be/professionelen/erkenningen/aanvraag/.
Offering food supplements in Belgium with a warehouse in the Netherlands
If you are a Dutch company with only a warehouse in the Netherlands and you want to offer food supplements in Belgium from here, you do not need a FAVV registration. You can apply for sales rights to sell food supplements in Belgium without providing a FAVV registration, by showing that your company is based in the Netherlands. However, you may only sell notified dietary supplements in Belgium.
How will your application be reviewed at bol.com?
In order to offer nutritional supplements to Belgian customers, you must therefore be able to prove that you are officially registered with the FAVV, or that your company is based in the Netherlands. For this, we need your FAVV registration number or business address.
Disinfectants
- For the sale of disinfectants, an authorisation policy is laid down by law. Consider items such as:
- Disinfectant hand gels
- Disinfection wipes
- Hand Alcohol
For this, it is a legal requirement to provide an authorisation number in the product information. You can enter them in the attributes Authorisation number NL and Authorisation number BE. If you only have the authorisation number for one country, you can enter a “0” for the other country. Please note: You cannot offer disinfectants for professional use on bol, not even with a valid approval number.
Bol.com checks whether the authorisation number appears in one of the following databases.
Second-hand & Refurbished
Second-hand
Second-hand items or margin goods can only be offered in the book, music and film categories. You cannot apply for selling rights for the other categories. It is therefore not possible to offer second-hand items other than books, music and films at bol.
Refurbished
The sale of refurbished range at bol has unfortunately been discontinued for the time being. This is because it is our responsibility to clearly show customers whether an item is refurbished, which is currently not technically possible. And we cannot solve this in the short term either. Refurbished items were allowed in a closed group until January 2022. It is not allowed to offer refurbished items.
Food and beverages for human consumption
To ensure that all food and drink products on our platform are safe and do not contain health risks for our customers, all legal requirements and regulations must be met. This applies to all consumer target groups.
Which items do we allow?
- Food products with a shelf life of at least 100 days after arrival at our customers’ premises.
- Foods for which there is an appropriate product category.
- Mandatory food information should be displayed on the label in the language of the destination country. This means at least in Dutch for items for the Dutch market and in French and Dutch for items for the Belgian market.
Which items do we not allow?
- Own-brand items from other supermarkets. For example, Albert Heijn private label items.
- Food to be refrigerated or frozen.
- Fresh or dried animal products intended for human consumption.
- Items, including compound food packages, where there is no mention of what exactly is in the package.
- Food and beverages in which CBD is present. CBD is only allowed in supplement form. See the CBD policy for more information.
- Alcoholic beverages with an alcohol content higher than 1.2%. See the Alcoholic beverages policy for more information.
In addition to the above guidelines that apply on the bol platform when selling food, you should also be aware of the legal obligations you need to comply with as a provider. Pay close attention to the following points:
- The GTIN/EAN of the item should be registered with GS1. Also, the full label information should be available in a GS1 GDSN data pool. This is GS1 Data Source (the Netherlands) or GS1 My Product Manager (Belgium). For more information, go to our page at GS1 Netherlands or here at GS1 Belgium.
- For articles containing animal ingredients, the manufacturer must be registered as a food business in the EU (issued by NVWA in the Netherlands or the FAVV in Belgium).
- Food products outside the EU will also need to be available in a GS1 GDSN data pool and meet the conditions for imported products outside the EU. More information is available here to find.
- Only approved food improvers and additives may be used in items. More information is available from the NVWA here.
Food supplements
Food supplements are allowed only if they comply with the obligations and guidelines above. Additional laws and regulations also apply:
- Food and beverage products may only contain permitted amounts of certain food supplements (and may not become a medicine). This also applies to food and drink products for specific consumer target groups, such as babies and toddlers, people with allergies or illnesses and people losing weight. This is subject to stricter rules on permitted amounts of certain food supplements.
- Please note that it is prohibited to claim on the label, packaging or advertising that the dietary supplement prevents, treats or cures any disease.
- Selling nutritional supplements in Belgium? See the self-care policy for more information.
Do you sell bulk packs?
Make sure you make this clear to the customer. For instance, by showing the article in bulk packaging in the main image and clearly stating ‘multipack’ or ‘bulk packaging’ in the product title. Also ensure that the product attribute ‘Packaging level’ is filled in, and that the official EAN is used that belongs to the bulk packaging (as registered with GS1).
Excise duty, deposits, VAT and levies
It is important to ensure that your items comply with obligations around excise duty, deposits, VAT and levies:
Deposit
- In the Netherlands there is a deposit on both small plastic ‘pet’ bottles (< 1 liter) and large plastic ‘pet’ bottles (>1 liter) soft drinks and water and from 01-04-23 there is also a deposit on (metal) beverage containers (cans). This amount, 0.15 cents for small plastic ‘pet’ bottles and 0.25 cents for large plastic ‘pet’ bottles, is charged at the time of purchase of the bottle and is returned upon return at a collection point. Bottles sold on the Dutch market must bear a deposit logo.
- From April 1, 2023, all metal beverage containers with a capacity of 3 liters or less must also bear a deposit logo and a new EAN code. These items must also be registered with Deposit Money Netherlands. The deposit obligation concerns cans for all types of beverages (such as soft drinks, water, milk and juice).
- The deposit obligation does not apply to cans with liquids that are not primarily intended to be drunk (such as lemonade syrup, thick juice, soups or condensed milk). Likewise, the deposit obligation does not apply to (canned) cans for non-liquids such as crisps, vegetables, etc.
- It is not possible to redeem deposit products at bol. More information about deposits is available here.
- There is currently no deposit on Belgian plastic bottles and metal drinking containers.
For this reason, it is not possible to offer these items without a deposit via bol on the Dutch market.
However, it is possible to offer these items without a deposit via bol on the Belgian market.
More information can be found on the website of Recycling Network Benelux.
Since August 1, 2023, the sale of cans without a deposit is no longer allowed in the Netherlands. Not even through bol. This is why all cans in the assortment are checked to see if they contain a deposit. To comply with the laws and regulations, cans of drinks without a deposit are taken offline in the Netherlands.
Excise duty
Production safety
To confirm that your items comply with guidelines and do not commit food fraud, among other things, we can request production documentation. This must demonstrate compliance with HACCP, such as the product manufacturer’s food safety plan and hygiene regulations. Once there is a serious suspicion that assortment is dangerous for the customer, your assortment can be (temporarily) offline while we assess the (potentially) dangerous items.
CBD
To safely offer items containing CBD (Cannabidiol) to our customers (and their pets), these items must comply with additional guidelines. CBD articles must comply with the conditions below, in addition to the applicable laws and regulations. CBD for human consumption is also required to comply with the Food and Drink Policy.
CBD for human consumption
- CBD items are only allowed in product group for supplements, as this is a food supplement.
- Food and beverages with added CBD are not allowed on platform. See here more information on the Food and Drink Policy.
- Selling CBD supplements in Belgium? See here more information on dietary supplements in selling Belgium.
Additional conditions:
- CBD articles should comply with the mandatory GDSN registration with GS1 required for food supplements. For more information on this GS1 registration in Belgium and the Netherlands.
- No active THC allowed, and it is mandatory to state the THC content. Listing an incorrect content is considered food fraud.
- You are not allowed to make medical claims, nor are you allowed to make health claims if they are not approved for the item in question. Please check whether your claim is allowed in the claims database.
- The item may not contain doses of active ingredients, making it subject to drug legislation.
- NOTE: Dietary supplements are subject to strict labelling requirements. These should be correctly labelled with additional information on the name and conditions of use of the item.
- When the product attribute ‘Type of herb or plant’ is filled in with ‘CBD’, it is mandatory to fill in the product attribute ‘THC content’ with a percentage.
CBD for animal consumption
For the safety of pets of our joint customers, it is important to comply with the following conditions:
- Only approved varieties of hemp are allowed in CBD articles for animals. See more information on this legislation here.
- It is mandatory to enter the full ingredients of the item in the product attribute provided. These should correspond to the actual content of the item.
Medical devices
To ensure that all medical devices on our platform are safe and contain no health risks for our customers, all legal requirements and regulations must be met. This applies to all consumer target groups. As a provider, you are always responsible for ensuring that your offer meets these requirements. Please note that the following guidelines apply on our platform when selling medical devices, but in addition to our guidelines, please be aware of the obligations that you as a provider must comply with.
What are medical devices and risk classifications?
A medical device is a product with a medical purpose for the user. This page has more information (in Dutch) on the definition of medical devices. Make sure you know well when your item is a medical device. Please note, for sales in Belgium, these selling rights still apply.
Each medical device has a risk classification associated with it, assessed for risk to the consumer. A risk classification must be present with every medical device. Risk classifications affect the product and the seller’s obligations. Based on the classification, various condition of the product will have to be met, such as having the product inspected by a notified body at medium or high risk class. Annex VIII of MDR 2017/745 contains the classification requirements for the risk class.
Legislation for medical devices
If you sell products in the EU that are or may be medical devices (including medical device accessories), you must comply with the MDR 2017/745. The MDR contains the legal requirements for medical devices.
Product safety
To confirm that your items comply with legislation, one of the things we can request is technical documentation demonstrating compliance with the MDR. Make sure you have this, along with your declaration of conformity, available at all times. As soon as there is a serious suspicion that an assortment is dangerous for the customer, your assortment may be (temporarily) offline while we assess the (potentially) dangerous items.
The European Quality Mark for Organic (Skal)
To ensure that our customers receive verified organic items, and we comply with the laws and regulations concerning organic items, we have set up a number of additional guidelines for the use of the Skal seal.
What is SKAL and what does it mean for sales of organic items?
SKAL is an independent administrative body that ensures that the production, processing and trading of organic items is done in a controlled and registered way.
Within the food, food supplements, animal food, plants and flower bulbs product groups, the term organic is a protected term. It may only be used for items that have been evaluated. You can recognise these items by the European Quality Mark for Organic, also known as the green leaf. The quality marks Agriculture Biologique, Demeter and EKO also meet SKAL’s requirements. The quality marks, therefore, may also display the green leaf on items. Any company that offers these products to our customers through our platform will have to register. Bol may only offer and trade in demonstrably certified organic items. This means that both the middleman and the producer must be certified.
Items must comply with:
What are the additional conditions for selling on our platform?
- Make sure your company is SKAL certified to trade organic items.
- Make sure your storage of items meets SKAL storage requirements.
- Apply for the ‘European Organic Label’,‘Agriculture Biologique’, ‘Demeter’ or ‘EKO’ sustainability label here so that your organic range is properly labelled on our website.
- The SKAL code NL-BIO-01 must be visible on the invoice to customers. To get the SKAL code on the invoice, it is important to put the SKAL code (NL-BIO-01) in the title of the organic product. By putting the code in the title, the code will automatically appear on the invoice to the customer. We recommend putting the code at the back of the title.
Cosmetics
To ensure that we offer safe cosmetic items to our joint customers, it is mandatory to comply with the applicable legislation for cosmetic products in EU, NL and BE. To comply with this, we have prepared the information and guidelines below. Please make sure you are aware of the legal requirements that apply to selling cosmetics.
What is a cosmetic?
“Any substances or mixtures intended to be brought into contact with the parts of the human body surface (epidermis, hair, hair, nails, lips and external genital organs) or with the teeth and the mucous membranes of the mouth, with the sole or principal purpose of cleaning them, perfuming them, changing their appearance and/or protecting or keeping the aforementioned parts of the body in good condition or correcting body odours” (Article 2.1a of the Cosmetics Regulation EC 1223/2009.).
Product safety and regulated ingredients
To ensure customer safety, the Cosmetics Regulation EC 1223/2009 requires every product to be assessed and found safe by a qualified safety assessor before it is sold. The responsible party (also called the Responsible Person/Responsible Person) is a person or company and must be able to make available a valid Cosmetic Product Safety Report (CPSR) from the safety assessor at all times. This must show that the product has been found safe by an authorised assessor and that the product meets all legal safety requirements. For confirmation, we can request this safety assessment from you, among other things. Cosmetics must be made and packaged according to certain hygiene standards (ISO 22716), this cannot just be done in the kitchen.
Once there is a serious suspicion that the range is dangerous for the customer, your range may be (temporarily) offline while we assess the (potentially) dangerous items. Responsible party of the article ensures that the correct documentation is available, when notification/assessment is involved.
Of course, it is not allowed to offer items containing banned ingredients, such as Lilial (BUTYLPHENYL METHYLPROPIONAL). Some substances are allowed in limited quantities. Cosmetics regulation EC 1223/2009 contains lists of:
- Prohibited substances (Annex II)
- Substances allowed in cosmetic products with set restrictions (Annexes III, IV, V and VI)
These lists and their restrictions change regularly. Make sure that the safety assessment (CPSR) remains up to date with changes in the product and with changes in legislation and thus that your products do not contain a recently banned substance or exceed recently (updated) restrictions. Also ensure that labels and online information are updated to the latest requirements regarding mandatory product information, instructions for use and warnings. Use the DIY Compliance manager to check whether ingredients comply with the latest legal restrictions and conditions.
Maximum concentrations for the substances Octocrylene and Benzophenone-3
Maximum concentrations for the substances Octocrylene and Benzophenone-3 apply. This means:
- Benzophenone-3 may be used up to a maximum concentration of 6% as a UV filter in products for face, hands and lips, excluding products in aerosols and spray pumps.
- Benzophenone-3 may be used up to a maximum concentration of 2% as a Uv filter in body products, including products in aerosols or spray pumps.
- In other products, Benzophenone-3 may be used as a Uv filter up to a maximum concentration of 0.5%.
- The use of Octocrylene as a Uv filter in products in aerosols is limited to a maximum concentration of 9%.
- The use of Octocrylene as a Uv filter in other products is limited to a maximum concentration of 10%.
In addition, products containing Benzophenone will be required to carry a warning label that reads, “Contains Benzophenone-3.”
Do you have any questions about the maximum concentrations of substances in your items? Please ask your supplier those questions. You can find more information on the EU-website.
Information on labels and online sales pages
For any cosmetic product, mandatory information about the product and its safe use must be available to consumers on the product itself and on the outer packaging and, in the case of online sales, also on the product’s sales page. If it is not possible to label the packaging, the option is to add a card with information.
Consumers (and authorities) should be able to see the following minimum mandatory information on the item, packaging and online sales page:
- Name and cosmetic function of the product (e.g. body lotion, baby shampoo, etc.)
- Ingredient list (in INCI nomenclature)
- Name and contact details of the Responsible Person in the EU
- Instructions and warnings for safe use (e.g. For hair colouring)
- Country of origin (if non-EU)
- Content (in g or ml)
- Period after opening (in months)
- In addition, the batch/lot code for traceability and shelf life should be mandatory on the label (but not online)
Make sure at least the mandatory product information (ingredient list, contents, period after opening and country of origin) is filled on the product page for your cosmetics range.
The list of ingredients must be displayed according to the international INCI naming standard. You must state the other information and instructions for use at least in the language(s) of the region where you market the product, i.e. in Dutch for products on the Dutch market and in Dutch and French for products on the Belgian market. More information can be found on Cosmetics Europe‘s website here. In addition, please ensure that the information is easy to read for our customers.
Ingredients obligation in INCI nomenclature
With cosmetics, it is compulsory to list the ingredients, for this you use the attribute ingredients. A clear and correct list of ingredients is compulsory, so that the composition of the cosmetic product is clear to everyone. The list should be drawn up in order of decreasing weight at the time of addition to the cosmetic product.
Ingredients must be listed with the correct INCI (International Nomenclature Cosmetic Ingredient) names. The European ingredient database Cosmile provides information on cosmetic ingredients.
Consumers with ingredient-specific allergies can therefore easily recognise ingredients, for example.
Cosmetic products registration
To sell a cosmetic product in the EU, it must have a responsible party (aka the Responsible Person/Responsible Person) with an address within the EU and the product must have been notified by the Responsible Person in the central EU system (Cosmetic Product Notification Portal, CPNP).
Do you make cosmetic products yourself? Or are you the person/company importing the products from outside into the European Union? Then according to the law, you are automatically the responsible party, unless this responsibility has already been taken over by another person/company (notifying party and indication on the label). If you are responsible yourself, make sure your products meet all the requirements AND that your products have been correctly notified (notified) in the CPNP. Non-notified cosmetic products may not be sold in the EU. To confirm that your items comply with the guidelines, we can request confirmation of CPNP registration from you, among other things.
Claims and advertising
For cosmetics products, both on the label and on online sales pages, no claims and advertisements may be made that violate the basic criteria described in Regulation 655/2013 and related directives.
This regulation states that claims must comply with all legal requirements and claims must not mislead the consumer or any other end user.
Basically, claims must meet the following criteria:
- Compliance with legal requirements
Failure to claim that a product complies with legal requirements - Correctness
If it is claimed that a product contains a particular ingredient, that ingredient must also be in the product.
Claims about the properties of a specific ingredient must not give the impression that the finished product also has those properties when it does not. - Evidence
Claims must be supported by sufficient and testable evidence - Fairness
It is not allowed to claim specific characteristics when similar products have the same characteristics.
When the effect of a product depends on its use in combination with another product, this must be clearly stated.
Claims must not go beyond what can be substantiated. - Biliqueness
Claims should be objective and should not be derogatory to ingredients or competition. - Making informed decisions
Claims should be clearly accurate, relevant and understandable to the average end-user.
Claims should also contain information that enables end-users to make an informed choice.
Instructions for completing the product information in your seller dashboard
Go to your sales account. Choose “Artikelen” and set a filter:
- on the above product groups at Productgroep/label and on
- Product information Zwak (offline) and Zwak (online)
PFAS
PFAS are chemicals with a number of properties. For instance, they are grease- and water-repellent. That is why PFAS are in many products. For example, in non-stick pans, raincoats, cosmetics and baking paper. PFAS are also found in fire-fighting foams, batteries and solar panels.
Bans on PFOS and PFOA, among others
There is a ban on some PFAS. For instance, PFOS has been banned in many applications since 2008. PFOA and some similar substances have been banned from consumer products such as non-stick pans since 2020. For more information, click here.
Any questions?
Chat with the partnerservice.
- Monday till Friday:
- 09.00 - 17.00